Which Best Describes the Second Law of Thermodynamics
Which statement best describes the second law of thermodynamics. Which best describes how the first and second laws of thermodynamics are related.
What Is The 2nd Law Of Thermodynamics Quora
Energy of the system decreases in a spontaneous process.

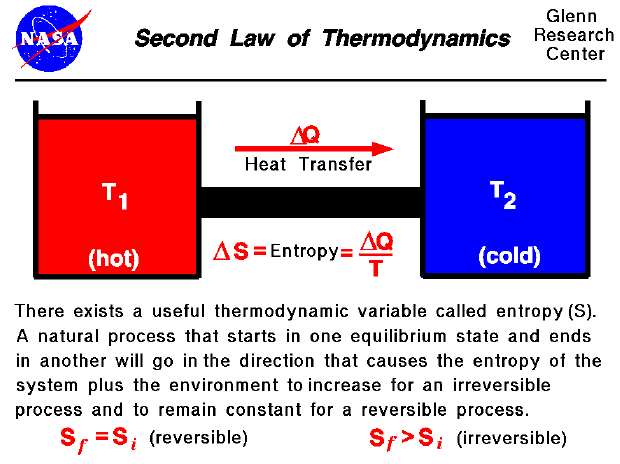
. The second law states that there exists a useful state variable called entropy S. Which best describes the second law of thermodynamics. ΔS univ 0.
Delta S delta Q T For a given physical process the combined entropy of the system and the environment remains a constant if the process can be reversed. Some thermal energy is lost to the surrounding environment as it travels through the system. Check all that apply.
An isolated system is best described by which one of the following statements. The time rate of change of entropy contained within a control volume is equal to the rate of entropy entering or exiting the control volume via heat transfer plus the rate of entropy entering or exiting the control volume via mass transfer plus the rate of entropy. Terms in this set 47 Which answer best describes the second law of thermodynamics as it is used in Engineering Thermodynamics.
The time rate of mass contained within a control volume is equal to the rate of mass entering the control volume minus the rate of mass leaving the control volume. High-temperature steam flows to an area of lower temperatures in the turbines. Isolated systems spontaneously evolve towards physics equilibrium the state with most entropy.
Entropy of the universe decreases in a spontaneous process. This happens once energy is transferred between trophic levels as illustrated in a very organic phenomenon. The entropy of a system always remains constant for a spontaneous process.
Which best describes the second law of thermodynamics. Chemistry questions and answers. Which answer best describes the second law of thermodynamics as it is used in Engineering Thermodynamics.
Thermal energy is used to do work on the turbines. The second law of thermodynamics is perhaps the most profound of the three laws of thermodynamics. A law stating that states that the entropy of an isolated system never decreases because isolated systems spontaneously evolve toward thermodynamic equilibriumthe state of maximum entropy.
Some useful energy is lost as heat whenever an energy transfer occurs. The second law of thermodynamics states that the total entropy of a system either increases or remains constant in any spontaneous process. Which of the following statements best describes the Second Law of Thermodynamics.
Thus the first law of thermodynamics is distinct from the second law as the former describes how thermal energy can be conserved to and from other forms of energy and not the direction in which it moves while the second law focuses on entropy. Where ΔS univ is the change in the entropy of the universe. What law is the law of entropy.
Thermal energy is used to do work on the turbines. The first law also known as Law of Conservation of Energy states that energy cannot be created or destroyed in an isolated system. Thermal energy is used to do work on the turbines.
Its importance is best expressed by sketching out a situation which violates it. B Energy can be neither created nor destroyed. Which observations correctly describe the second law of thermodynamics.
The second law of thermodynamics states that the full entropy of the associated isolated. It can be considered as a quantitative index that describes the quality of energy. Entropy is a measure of the randomness of the system or it is the measure of energy or chaos within an isolated system.
The second law of thermodynamics. During respiration some energy is lost as heat. Mathematically the second law of thermodynamics is represented as.
The second law of thermodynamics states that whenever energy is remodeled theres a loss of energy through the discharge of heat. 1 3 4 5. The change in entropy delta S is equal to the heat transfer delta Q divided by the temperature T.
Which choice describes the second law of thermodynamics best. The time rate of change of energy contained within a control volume is equal. 1 Point In all ecosystems energy is neither created nor destroyed but it may be converted from one form to another.
Which of the following statements best describes the Second Law of Thermodynamics. Equivalently perpetual motion machines of the second kind are impossible. A The internal energy of the universe is constant.
The Second Law of Thermodynamics states that in all energy exchanges if no energy enters or leaves the system the potential energy of the state will always be less than that of the initial state. A The entropy of the universe must increase for any spontaneous process. Energy of the system increases in a spontaneous process.
Energy transfers are always 100 efficient. The entropy of the universe increases in a spontaneous process. Energy cannot leave a system.
Energy can be created from matter or used to produce matter. High-temperature steam flows to an area of lower temperatures in the turbines. This best describes the second law of thermodynamics which states that Some useful energy is lost as heat whenever an energy transfer occurs.
B The entropy of the system mustincrease for any spontaneous process. This is also commonly referred to as entropy. C The entropy of the surroundings must increase for any spontaneous process.

Solved Which Best Describes The Second Law Of Chegg Com

0 Response to "Which Best Describes the Second Law of Thermodynamics"
Post a Comment